Research Areas

Nanoporous materials for challenging separations
Separation processes alone accounts for about 15 % of global energy consumption. Our research focus on those separations in which conventional techniques like distillation are extremely energy intensive while separation using polymeric membranes are not efficient. Examples include propylene/propane, ethylene/ethane separation etc. Our approach involves the use of the recent developments in the field of nanoporous materials to address these challenging separations. In particular, we use metal organic framework based membranes and engineer their properties to maximize the separation efficiency.
Keywords: Olefin/Paraffin, MOFs, Membranes



Carbon Capture
Increasing concentration of CO2 in the atmosphere is the main cause for global warming and associated climate change. Capture of CO2 from point sources like steel, cement and power plants, is identified as the top priority in curbing the emissions. Our research focuses on the development of adsorption or membrane-based techniques for pre/post-combustion capture of CO2.
Keywords: Adsorption, Membranes, Pre-Combustion, Flue Gas, Climate Mitigation


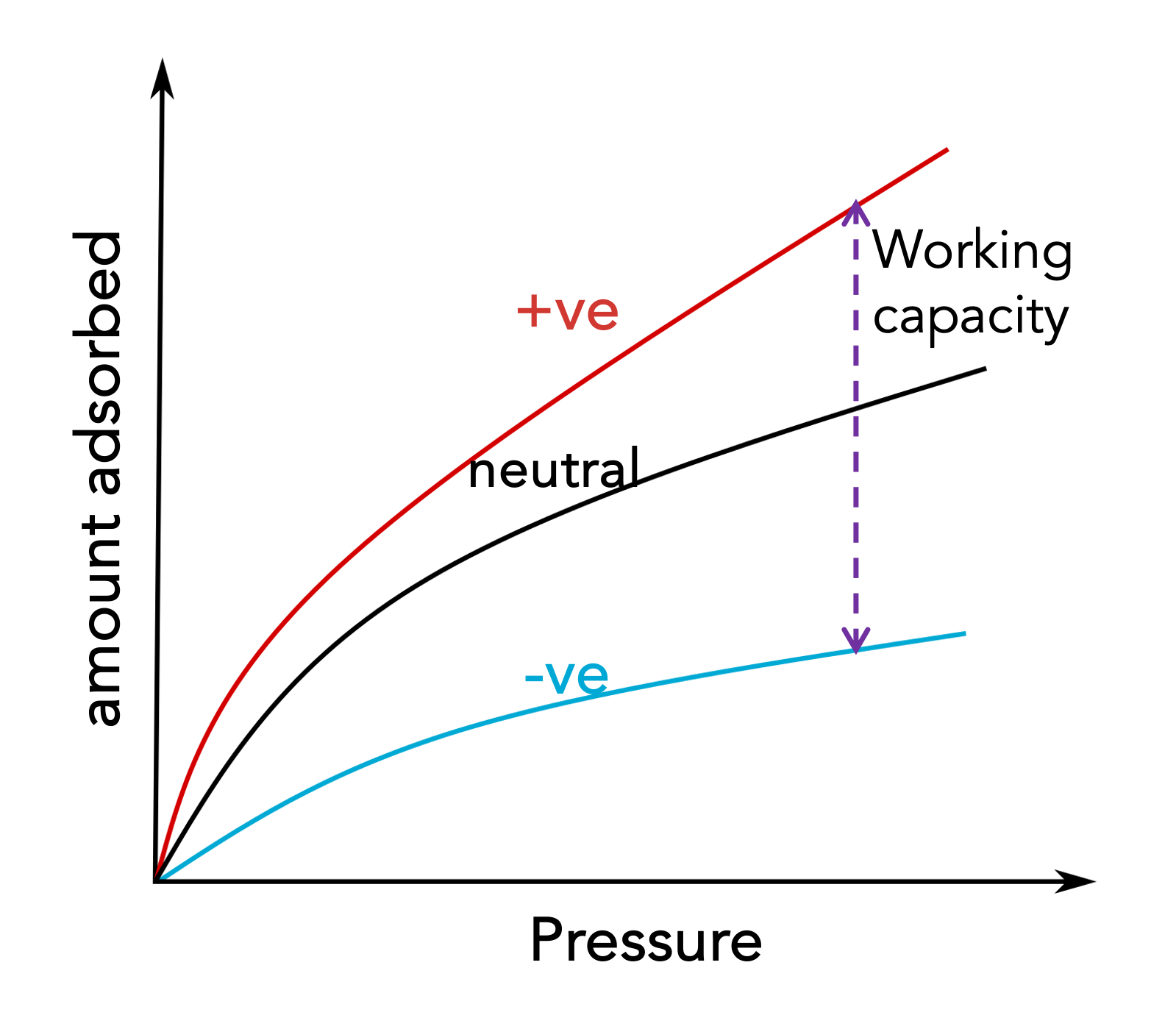
Electric swing adsorption
Conventional sorption technology for the separation of gases relies on temperature or pressure swings for modulating the affinity of the adsorbent species. A significant amount of energy is consumed in the sorption-regeneration cycle. Electric swing adsorption (ESA) is an emerging area of research, where adsorption and desorption can be brought about by switching the polarity. Being an isothermal process and no cyclic pressurizing or depressurizing is involved, a significant amount of energy can be saved in the capture process. ESA is still in its nascent stage and our research focuses on developing ESA as a viable technology for practical separation applications.
Keywords: Adsorption, Gas Separation, Carbon Capture, Redox-Active



Nucleation and crystallization studies
Control over nucleation and growth are fundamental for engineering the properties of a material. Our research focuses on understanding the nucleation and growth characteristics of porous materials on different 2D substrates like graphene and MoS2 using both in-situ (liquid cell TEM) and ex-situ approaches.
Keywords: Nucleation, Single Crystal, In-situ Microscopy, MOF, Graphene, MoS2


Chemical Vapor Deposition
CVD is a versatile technique for producing thin films, coatings or nanomaterials on a substrate surface by chemical reactions that occur in the vapor phase. Currently our research focuses on the synthesis of monolayers of 2D materials like graphene or transition metal dichalcogenides (TMDs) like MoS2 or WS2 or their heterostructures for various applications. Both atmospheric pressure CVD and low pressure CVD techniques are pursued for synthesizing atomically thin 2D materials on different substrates including glass.
Keywords: Graphene, MOS2, WS2, Glass, Low-Temperature Synthesis, 2D Materials, Membranes


Graphdiyne
Graphene is often considered as the ultimate membrane material due to its single
atom thickness offering very high theoretical permeance. However, pristine graphene is impermeable even
to helium necessitating pore generation approaches. Graphdiyne (GDY), an sp - sp2 allotrope of carbon
and a member of the graphyne family, are also single atom thick with a 2D structure. GDY has uniformly
distributed high density of sub-4 Å pores which makes them very interesting for gas separation
applications. Our research focuses on the synthesis and development of GDY membranes for various
separation applications.
Keywords: Graphynes, Graphdiyne, 2D Materials, Membranes




Active sieving
Through millions of years of evolution, nature has perfected the art of separation.
Nature seldom relies on a single mechanism for separation of different species but uses a synergistic
combination of two or more different mechanisms. A classic example is aquaporins in kidneys which uses a
combination of size-sieving, charge separation and hydrophobicity to achieve unparalleled water
filtration. Inspired by this, we aim to develop separation technologies with a synergistic combination
of multiple separation mechanisms with at least one activated by an external stimuli.
Keywords: Stimuli Responsive, Size Sieving, Rectification, Biomimicking


